The STARprobeTM
A new technology allowing simultaneous measurements
of four cryolitic bath properties in only four minutes
1Pierre Bouchard, Eng., M.Sc., President
STAS inc., 1846, rue des Outardes, Chicoutimi (Quebec), Canada G7K 1H1
2Dr. Marc Dupuis
GeniSim Inc., 3111, rue Alger, Jonquière (Québec), Canada G7S 2M9
During the last 10 years, Alcoa has developed a new device called the STARprobe™ [1,2,3].
This technology, now available through STAS, is a portable device that takes real time
measurements of four bath properties in electrolysis cells:
Superheat
Temperature
Alumina concentration
Ratio (excess AlF3)
(STAR)
This synchronicity of measurements is the most important step forward in improving the control
and efficiency of electrolysis cells.
To effectively control an operating cell and to achieve its maximum efficiency, energy state,
chemical state, and state of control should be known. These states are represented by core
parameters including cryolite ratio (%XS AlF3), temperature, superheat and concentration of
alumina.
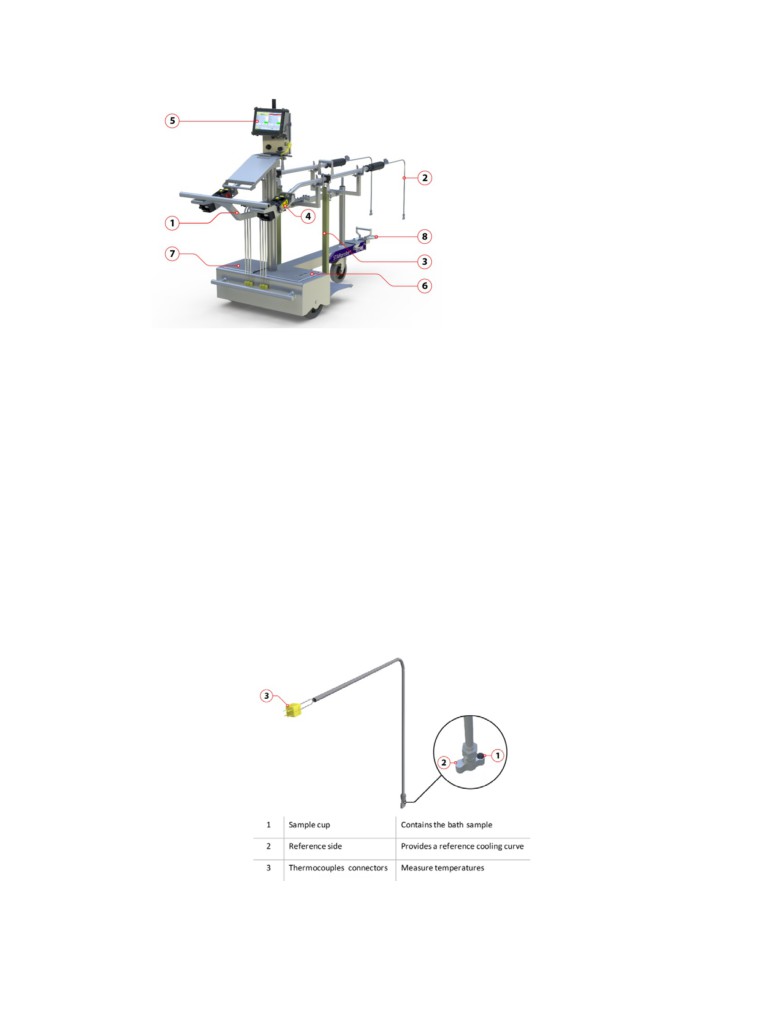
Figure 1 shows one STARprobe™. The device kit consists of a mobile cart and two probes, one
interface computer, one power unit, one battery pack and a telescopic towing arm in the front.
The first step to achieve optimum control is to measure the parameters properly.
Bath chemistry and cell operating temperature in aluminium cells must be controlled to achieve
optimal current and energy efficiency. Up until now, the conventional way to control the bath
ratio and temperature has been to regularly take bath samples for chemistry analysis, and the bath
temperature is measured directly in the pot. These measurements are generally performed
separately, typically with a difference of 24 hours. Moreover, bath samples have to be sent to a
laboratory for analysis, with results available in as much as 24 hours later. Due to the delay in
getting the bath sample analysis results, control decisions have to be made primarily relying on
old and out of sync information. This leads to an unsteady feedback control loop, where the cell
is continuously under or over shooting the targeted optimum conditions, which causes
sub-optimal cell performance in terms of both current and energy efficiency.
1
1- Cart
2- Reusable probe tip
3- Probe
4- Electronic probe head
5- Interface computer
6- Power unit
7- Battery pack
8- Telescopic towing arm
Figure 1: STARprobeTM device kit
In addition to drawbacks of the usual measurement methods, there is also lack of some key bath
physicochemical information, such as bath superheat temperature that is critical to efficient
operation. Some measurement methods have been a subject of study in the past decades. The
commercially available measurement tool by Electronite, made it possible to utilize measured
bath superheat for active pot control. Though it was a step forward from the traditional method, it
never reached large scale application across aluminum smelters.
The new STARprobeTM provides accurate, inexpensive and perfect synchronicity of measurement
of the four basic cell operating parameters.
Principle of Operation
The probe concept consists in making a Differential Thermal Analysis (DTA), which is a proven
method [4], on a bath sample and a reference. The reusable probe tip (Figure 2) includes two
high-precision type K thermocouples, dynamically paired for compatibility.
Figure 2: Reusable probe tip
2
The thermocouple on the right records the cooling rate of the bath sample, while the
thermocouple on the left records the cooling rate of the reference.
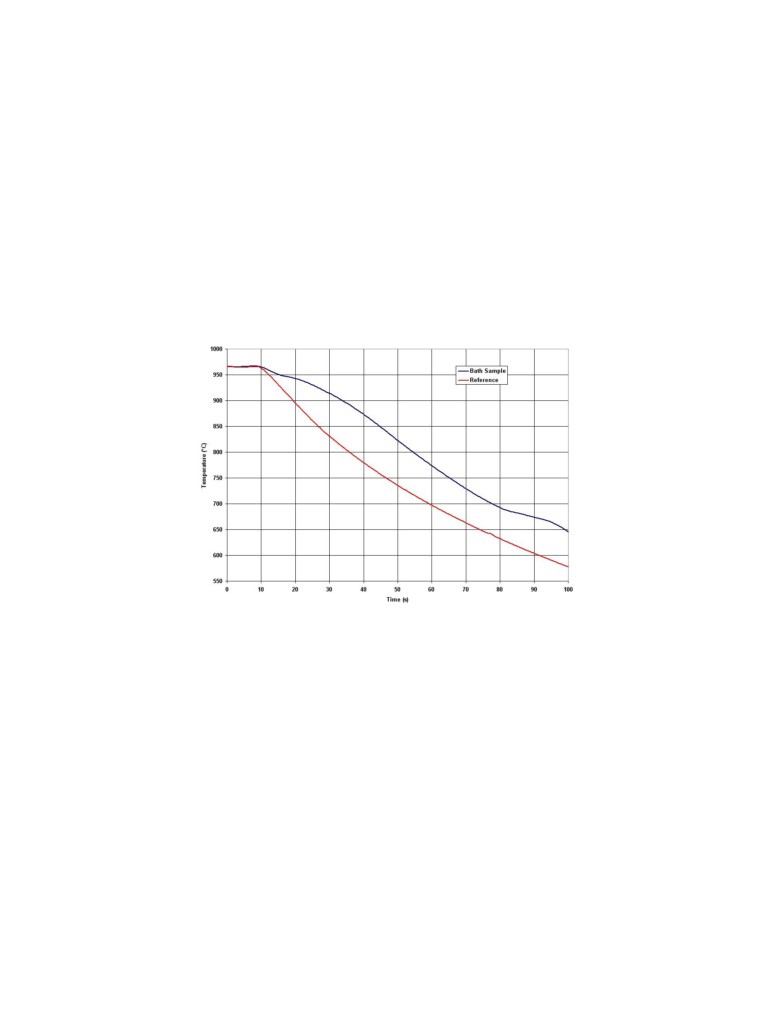
Figure 3 presents a pair of DTA
(Differential Thermal Analysis) curves obtained from a
STARprobeTM measurement. The cooling rate of the bath sample is slower than the metallic mass
of the probe for two reasons. The first and less significant reason is because of the difference of
thermal diffusivity between the bath sample (liquid and solid) and the metallic mass of the probe,
hence the initial separation of the two curves between 10 and 18 seconds. Second, at the bath
sample liquidus temperature, cryolite starts to solidify, which slows the bath sample cooling rate
down even further. At the cryolithe-alumina phase diagram bath eutectic temperature, the
alumina starts to solidify as well. Finally, at a much lower temperature (at the cryolite-AlF3 phase
bath eutectic temperature), the excess AlF3 finally solidifies [5].
Figure 3: Recorded cooling rate of bath sample and the metallic mass
of the probe, which act as reference temperatures in the DTA
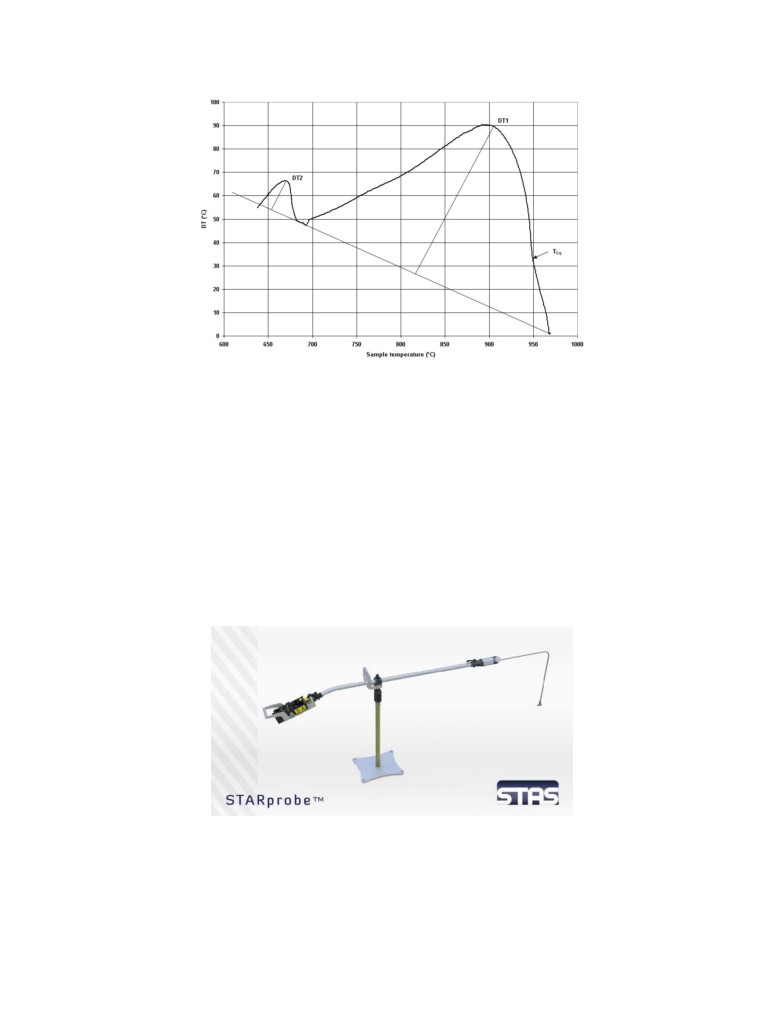
The difference of temperature between the two curves is computed and presented on a second
graph (Figure 4). In this case, the sample temperature is selected as an X coordinate. The shape of
that curve is independent of the cooling rate, so the bath sample analysis results will not be
affected by fluctuation of the ambient conditions [1]. In fact, the shape of the curve depends only
on two things, the design of the probe tip and the composition of the bath sample. This means
that for a given probe tip design, the shape of the curve uniquely depends on the composition of
the bath sample. This is the reason Alcoa was able to come up with a correlation algorithm that
could identify the bath composition from the shape of each curve measured. The high
temperature maximum is due to the solidification of the cryolite, while the low temperature
maximum is mainly due to the solidification of the excess AlF3. The more AlF3 in the collected
bath sample, the less intense the high temperature peak will be and the more intense the low
temperature peak [5].
3
Figure 4: Differential temperature curve
and one possible way to perform the DTA analysis
How it is used in the potroom
The reusable, consumable probe tip can take around 100 measurements. It is connected to the
probe head through a probe lance, as seen in Figure 5. An operator can use two of those
assemblies to measure cells simultaneously. That way, a trained operator can obtain an average
time of just under 4 minutes per measurement. Each measurement is done in three steps:
1- insertion of probe into molten bath to equilibrate with bath temperature in the pot cell;
2- removal of probe tip from bath, and cooling of probe tip;
3- analysis of cooling curve by STARprobeTM and recording of results.
Figure 5: Probe assembly
The probe head includes a very high-resolution electronic thermocouple reader that reads the
thermocouples and transmits data via Wi-Fi to the tablet PC running the STARprobeTM
application. One tablet PC can simultaneously process the data from the two probe heads. After a
4
few seconds, the results are displayed on the tablet screen (left side of Figure 6), stored in a file
on the tablet and can be transmitted automatically to the plant database and/or to the pot control
system.
Probe 2A
Probe 2B
Figure 6: STARprobeTM application displaying the results
Potential of process control improvement using the STARprobeTM
In most plants, the way to control the bath ratio and the temperature is usually to take bath
samples regularly and to measure the bath temperature. Most of the time, bath sampling is not
synchronized with bath temperature measurement. In any case, due to the delay in getting the
bath sample analyzed, the cell controller typically never receives new temperature and ratio data
at the same time. This lack of synchronicity between the bath ratio and bath temperature data and
the lag in getting the ratio data is totally eliminated by using the STARprobe™ to measure both
parameters at the same time and by immediately transmitting the results to the database and to the
pot control system via Wi-Fi.
Furthermore, a typical bath ratio control logic uses the data for both the bath temperature and
bath ratio in order to control the bath ratio by adjusting the amount of AlF3 added to the cell,
assuming a given and constant cell superheat. As presented in [7], any inconsistencies between
the target bath ratio and the target bath temperature can create instabilities in the feedback control
loop.
Since the STARprobeTM also measures the bath superheat, the bath ratio control and the bath
temperature control - or rather the bath superheat control - can be decoupled. The bath ratio can
be controlled by adjusting the amount of AlF3 and the bath superheat by adjusting the target cell
pseudo-resistance independently [8].
Improvements have already been achieved in more than 10 of Alcoa’s plants in terms of process
control. In parallel with the development of the STARprobe™, Alcoa has developed a new cell
controller called QLC which takes full advantage of its STARprobe™ bath properties
5
measurement technology. The QLC automatically acquires the results of STARprobe™
measurements in real time via Wi-Fi [1,6].
The gains guaranteed by using an expert pot controller are the following:
• 0.5% current efficiency (proven)
• 35 mV voltage savings (proven)
• 5% AlF3 savings (proven)
• 100-150 day potlife improvement (still to be established)
• One time capital cost saving (X-ray equipment) (proven)
• Labor savings for sampling/analysis (proven)
• Improved understanding by operators (proven).
The STARprobe™ technology is a new way to control electrolysis cells. Other companies around
the world have already taken advantage of this new opportunity.
References
[1] Xiangwen Wang, Bob Hosler and Gary Tarcy. Alcoa STARprobe™, Light Metals, (2011),
483-489.
[2] Bob Hosler, Xiangwen Wang, Jay Bruggeman and Patrick O’Connor. Molten Cryolytic Bath
Probe, US patent no. 2005/0069018 A1.
[3] Xiangwen Wang, Bob Hosler and Gary Tarcy. Systems and Methods Useful in Controlling
Operation of Metal Electrolysis Cells, US patent no. 2007/0295615 A1.
[4] R.C. Mackenzie. Differential Thermal Analysis, Academic press London, 1970.
[5] Dr. Marc Dupuis. Bath Ratio and Temperature Control Enhancement in the Potroom, Light
Metal Age, October 2013, p.61.
[6] Wang, X., Tarcy, G., Batista, E. and Wood, G. Active pot control using Alcoa
STARprobe™, Light Metals, (2011), 491-496.
[7] M. Dupuis. Excess AlF3 concentration in bath control logic, National Conference on
Advancements in Aluminium Electrolysis, Indian Institute of Metals, Angul Chapter, (2006).
[8] M. Dupuis and J-P. Gagné. Testing a new STARprobe™, Aluminium 89 (2013) 1/2, 76-79.
6